This is a guide to antibiotics, main classes and how they work. there will be a slide at the bottom for general use.
Antibiotics work on the principle of differential toxicity. They are toxic to bacteria but not to our cells. This is because the antibiotic target is either not present in eukaryotic cells or is sufficiently different as to not be greatl effected.
Sometimes bacteria can figure out how to live with them and that will be discussed in Antimicrobial resistance
The main classes of antibiotics are grouped by mechanism of action:
Cell wall synthesis
Betalactams
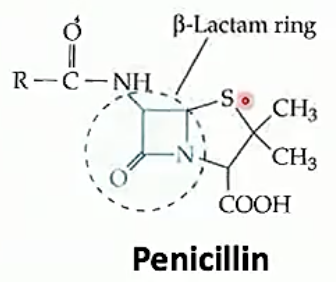
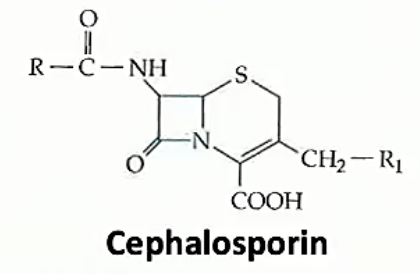
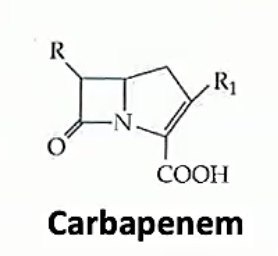
Betalactams are all related by the beta lactam ring, a square ring which covalently bind to the active site of a protein which fuses components together to make bacterial walls. This means that the bacterial walls are weak and the bacteria has a hard time growing if at all. Beta lactams differ in the side chains and stuff other then the lactam ring. Different ß-lactams have different pharmacological properties and bacteria have differing resistances to each
The ß-lactams stop peptidoglycan from being cross linked to other peptidoglycan chains by inhibiting proteins which bind polypeptide side chains and covalently bond them to one another
Allergy to penicillins: Allergy to penicillin is overreported and often it is to a side chain and not the ß-lactam ring. (but proper allergy can occur)
- 2.5% cross reactivity of penicillin allergy with cephalosporins
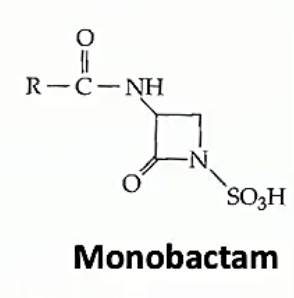
- <1% with carbapenem and monobactams
Sub-classes are:
-
Penicillins
-
Cephalosporins
-
Carbapenems
-
Monobactams
Bacitracin
Glycopeptides
These are massive molecules which are unable to cross the gram negative lipid barrier.
These work by covering the peptidoglycan side chains like a glove so they cannot by fused and cross linked
Examples are:
-
Vancomycin
-
Teicoplanin
Cell membrane
-
Polymyxins
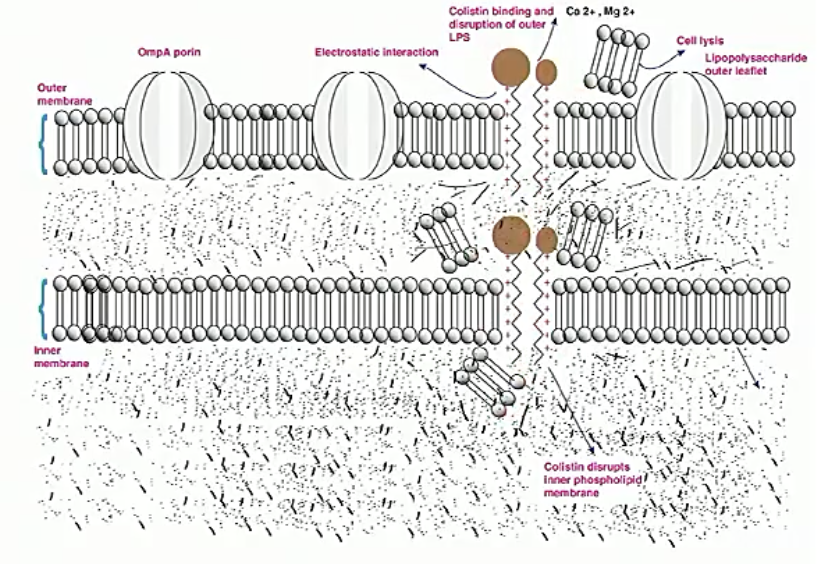
These are drugs of last resort (polymyxins are toxic) and they insert and lyse bacterial membranes.
These are only for gram negatives
Examples: colistin
-
Daptomycin
This also bind to membrane and punches holes in membrane These are gram positive only
Nucleic acid synthesis
Folate synthesis
These work by inhibiting the bacterial folate synthesis pathway. Folate is vital for DNA synthesis. The tetrahydrofolic acid pathway is multistep and therefore has multiple sites to attack. Both sulfonamides and trimethoprim are analogues to intermediaries
-
Sulfonamides
-
Trimethoprim
-
Co-trimoxazole (sulfamethoxazole and trimethoprim)
RNA Polymerase
Resistance to this can happen through a single point mutation
This targets RNA polymerase.
Examples:
-
Rifampin
This is an important drug for treating TB
DNA Gyrase
Circular DNA chromesomed often need to twist and untwist (like holding a rubber band at 2 points and rolling one half inbetween your fingers). type II topoisomerase introduce negative supercoils. this counteracts the positive supercoils introduced by dna replication and if inhibited DNA reproduction is hampered
Examples:
-
Fluoroquinines
Metronidazole
This is a prodrug which is converted to a highly toxic drug when it reacts with Ferredoxin/Flavodoxin which is an electron carrier in anaerobes (the equivelent of NAD+/NADH)
Protein synthesis
These all work by binding to the bacterial ribosome. They can bind to the large (50s) or small (50S) subunits.
differential toxicity exists as the bacterial ribosome is different from outs
30s subunit small subunit
Inhibit the mRNA
-
Tetracyclines
-
Aminoglycosides
50s subunit large subunit
Inhibit the tRNA
-
Macrolides
-
Clindamycin
-
Linezolid
-
Chloramphenicol
-
Streptogramins
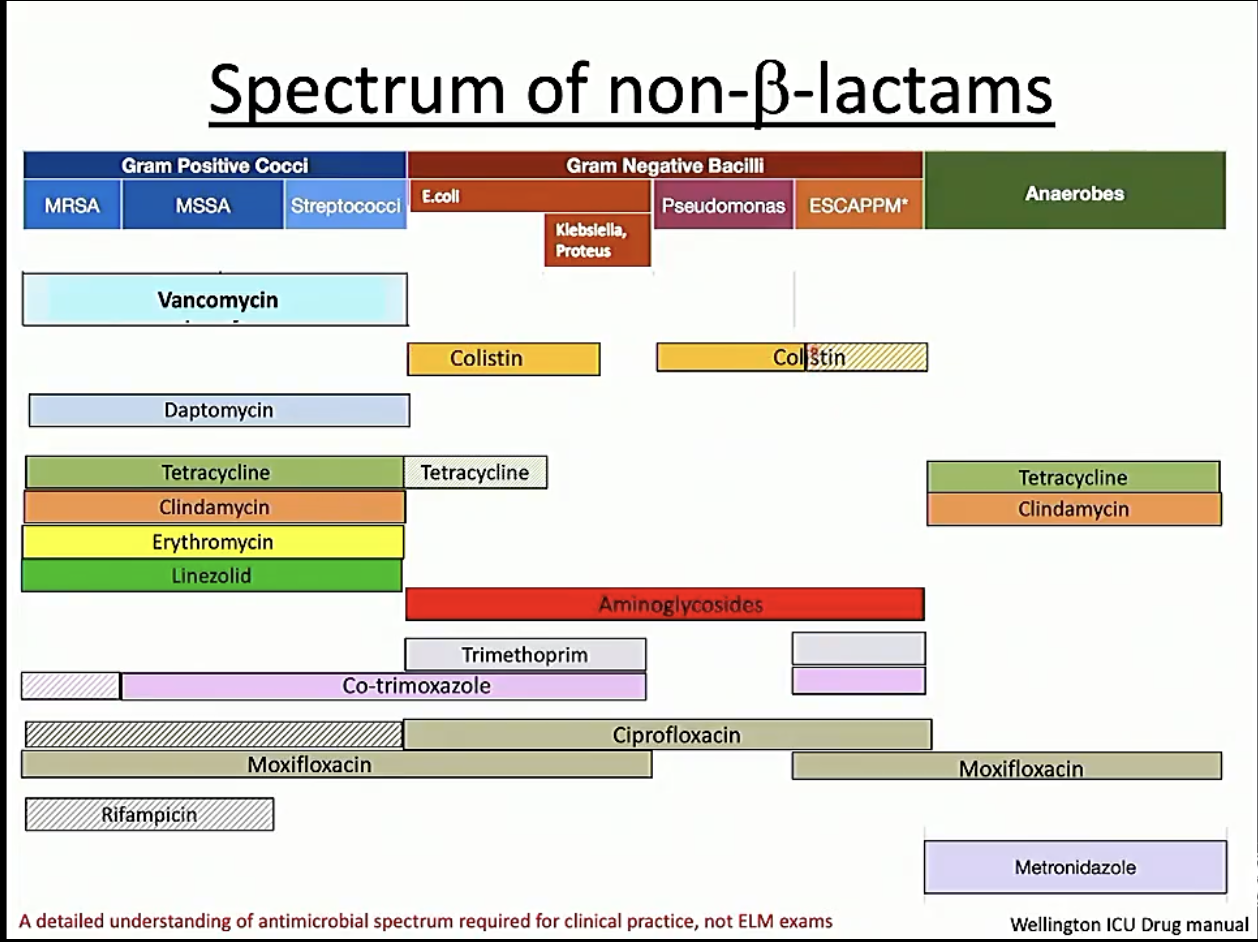
ß-lactam susceptability
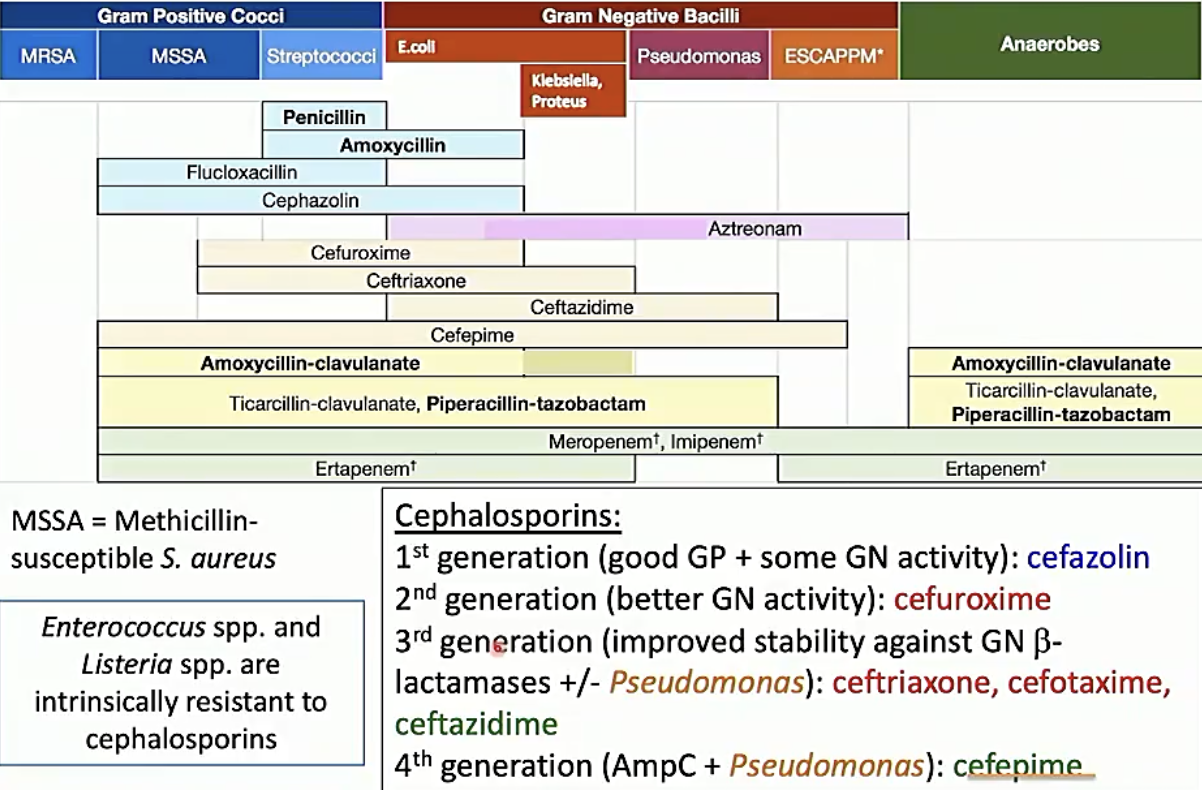
Non ß-lactam susceptability