Oxygen transport
Blood is transported in 2 forms, dissolved as gas (low) and combined with haemoglobin.
Dissolved
Very low amount is carried as dissolved O2. Therefore most is carried bound to haemoglobin.
Carried with haemoglobin
Oxygen reversibly binds well to haemoglobin to make oxyhaemoglobin. the binding is dependant of the PO2. The disassociation curve that results is sigmoidal due to the fact that the O2 binding of one subunit in the tetramer influences the others. additionally the tetramer can have its binding properties altered by metabolic products (BPG) and carbon dioxide through acidity.
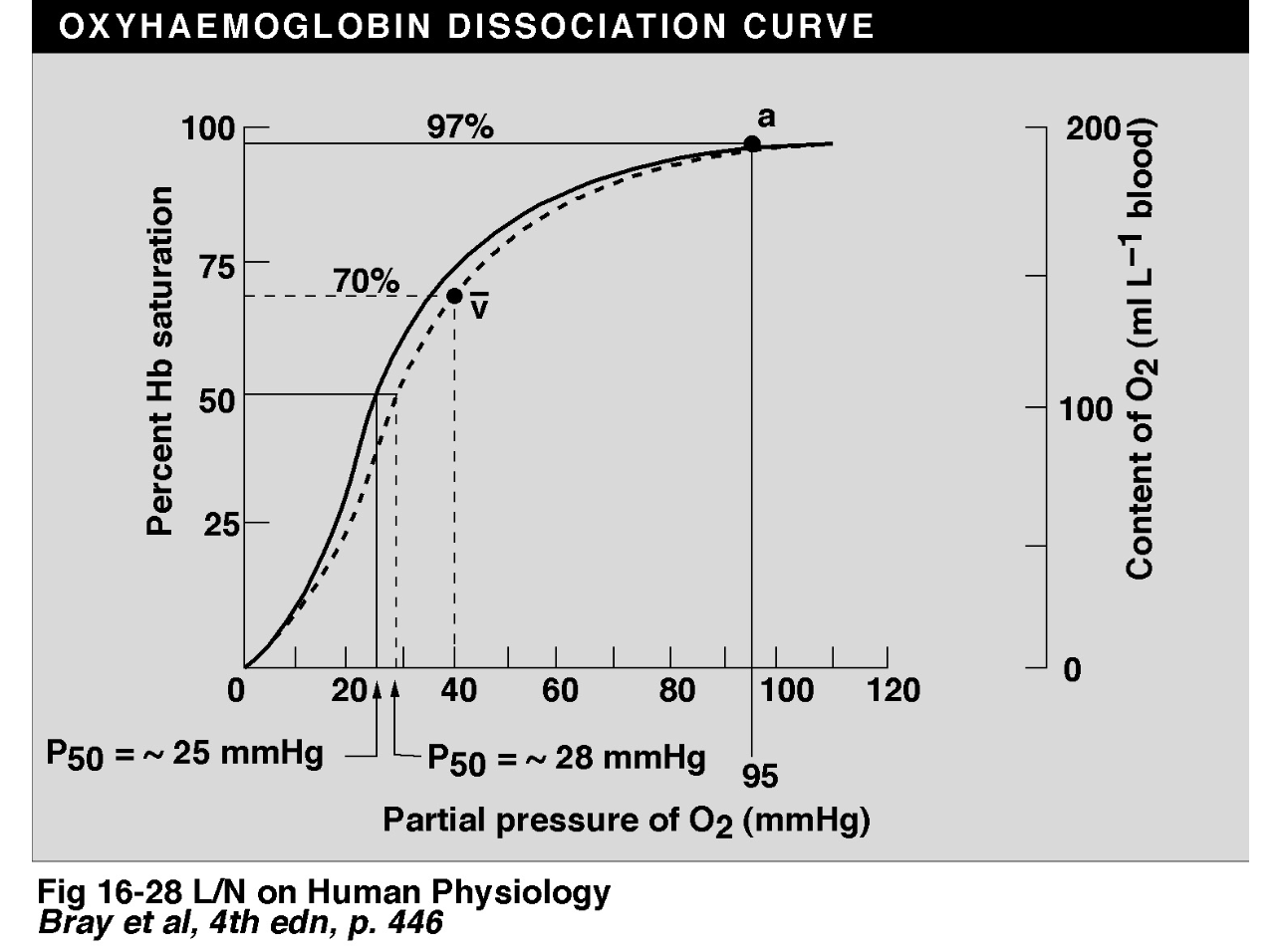 The sigmoidal curve offers advantages: the flat upper bit allows the PO2 to change a little and not have massive effects on the Hb saturation percentage and the steep lower bit allows oxygen disassociation to tissues that need oxygen
The sigmoidal curve offers advantages: the flat upper bit allows the PO2 to change a little and not have massive effects on the Hb saturation percentage and the steep lower bit allows oxygen disassociation to tissues that need oxygen
Hb capacitance for O2
matty b here decides to enlighten us on how to calculate the hb capacitance for O2 which i find to be rather dry and will give you the final number. 200 ml of O2 per litre blood is the arterial content (roughly)
Shifts in the oxygen disassociated curve
Leftward shifts in the graphs line means more loading of O2 in the lungs, a rightward shift in the graphs line means more unloading of O2 into the tissues. This lovely diagram displays this: 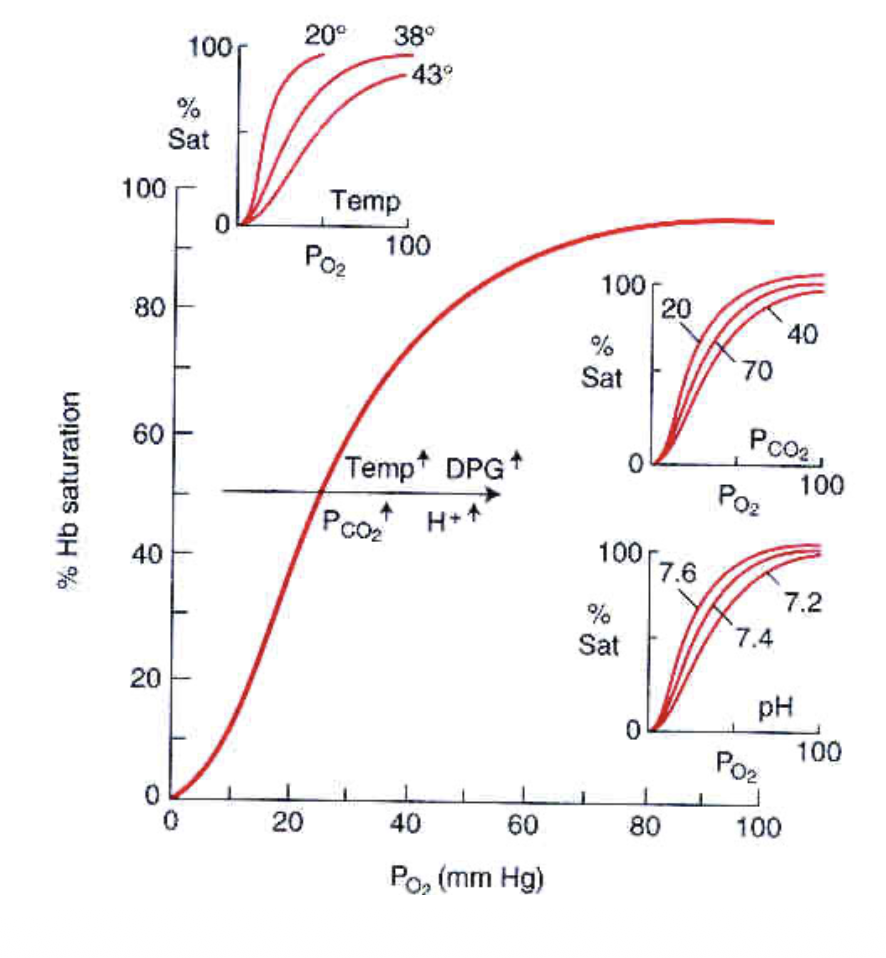
The Bohr effect
This is a rightward shift (favouring disassociation) in tissues which need more oxygen. In the presence of increased:
- PCO2
- H+ concentration
- temperature
- 2,3-diphophoglycerate in RBCs
A subsequent good example for this is that in active muscle there is heat, hypercapnia, and acidity, and you need oxygen in these regions.
What is 2,3 DPG?
2,3 DPG is a by-product of glycolysis in RBCs. it is usually consumed by the mitochondria but RBCs don’t have any. it increases with intense exercise training, altitude, and due to severe lung diseases or anaemia.
Conditions
Cyanosis
Cyanosis is visible when there is deoxygenated blood in a tissue, visibility depends on a number of factors, but often the presence of cyanosis is detectable when there is at least 50 g/L of deoxyhaemoglobin.
Anaemia
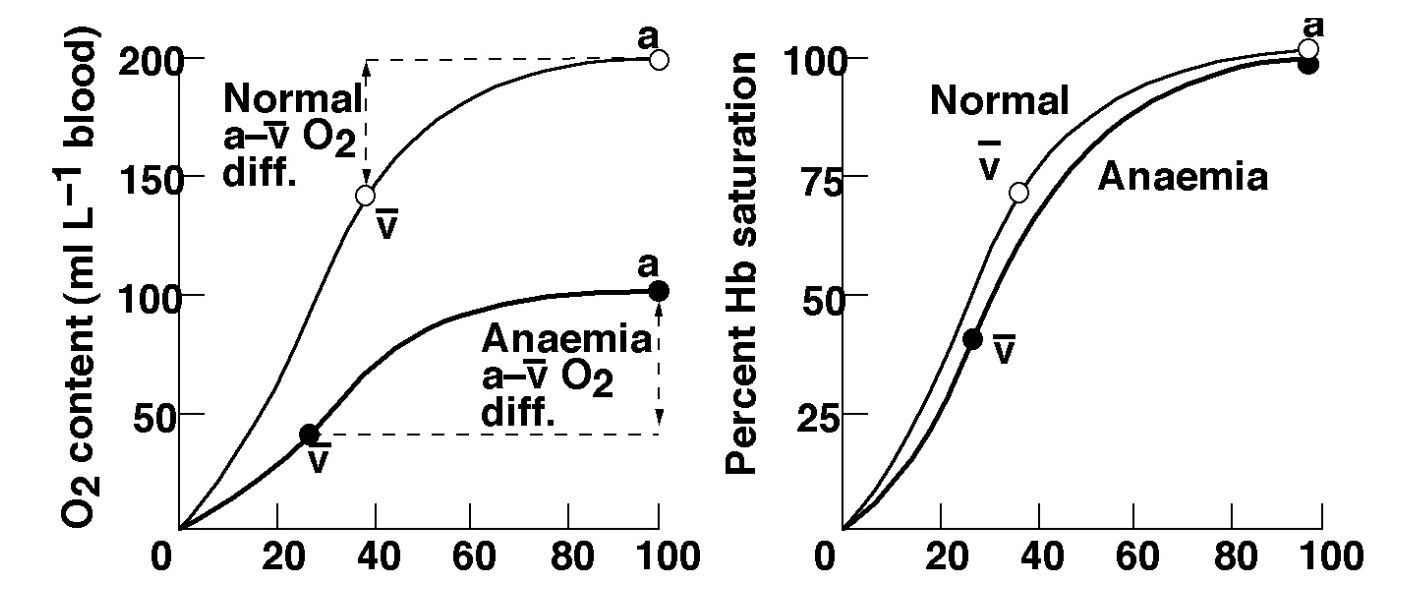
Anaemia reduced the amount of O2 carriers in the blood. subsequently the percent Hb saturation is unchanged, However the O2 concentration is. (lecture slides state exercise problems from a-v difference but i need to recheck) These graphs shows differences nicely (pay attention to Y-axises)
Carbon monoxide 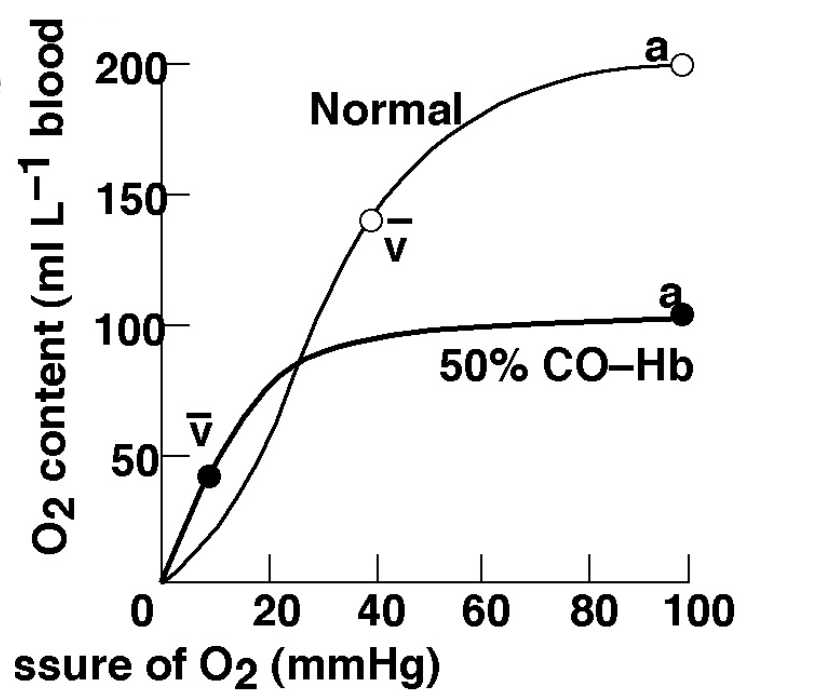
Carbon monoxide LOOOOOVES haemoglobin and binds to it very strongly. this limits the amount of Hb binding sites for oxygen. Additionally small amount of carbon monoxide can greatly impair the blood ability to carry oxygen. This results in a massive rightward shift which makes unloading of O2 very difficult.
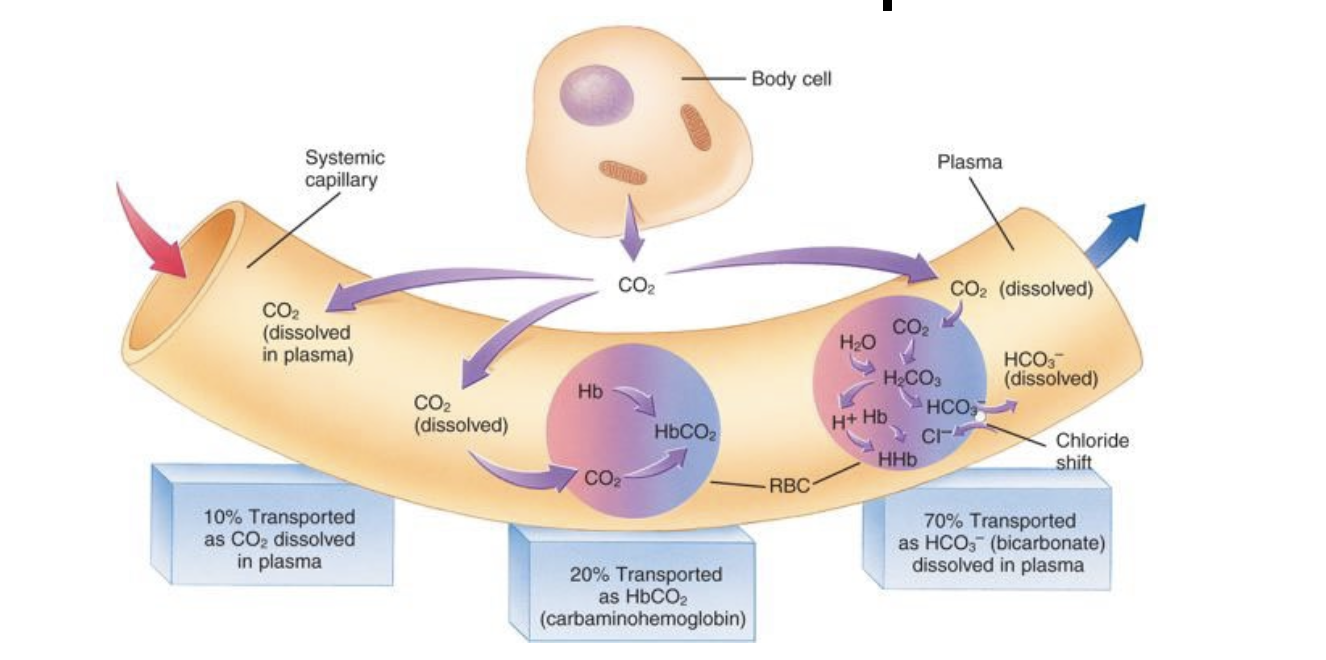
Carbon dioxide transport
Carbon dioxide is transported in 3 ways:
- Dissolved in plasma (10%)
- as bicarbonate (70%)
- combined with proteins as carbamino compounds (20%) (This is carboxyhaemoglobin i think)
This image sums it up nicely: 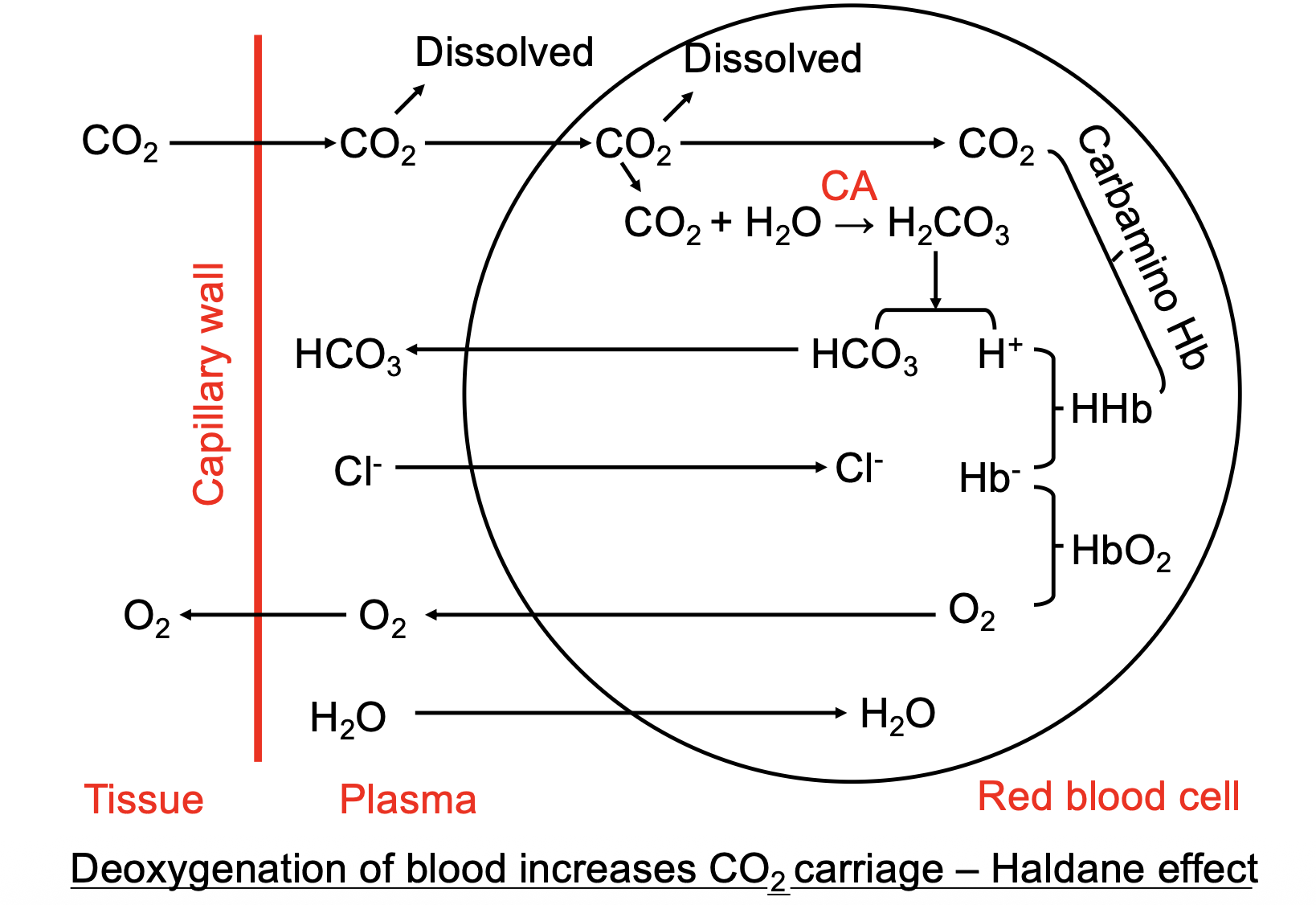
 Haldane vs bohr effect
Haldane vs bohr effect
Lungs Haldane is Hb oxygenation leads to CO2 unloading Bohr is decreased CO2 leads to O2 loading
Tissues Bohr is increased CO2 faciliates O2 loading Haldane is unoxygenated Hb faciliates CO2 loading
Effects of changes in carbon dioxide blood levels
btw you produce around 10mmol/min CO2
Hyperventilation → more CO2 released from body than normal → hypocapnia hypoventilation → less CO2 released from body than normal → hypercapnia ---and then--- hypercapnia → more carbonic acid formed than usual → Respiratory acidosis Hypocapnia → less bicarb ions formed then usual → Respiratory alkalosis
Compensation
Changes in blood pH is quite bad so your body has a system of regulating changes in blood pH. the kidneys and the lungs work in tandem to try to both cover each other and other sources of pH variation. The kidneys achieve this by secreting more H+ ions and making more bicarb (basic), whereas the lungs will ventilate more or less to try to bring pH to normal physiological levels.
The renal system will compensate for respiratory acidosis or alilosis and the respiratory system will compensate for metabolic acidosis (eg diabetic ketoacidosis)